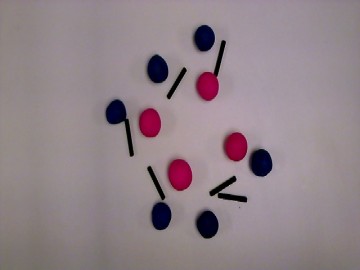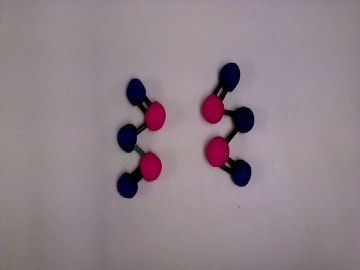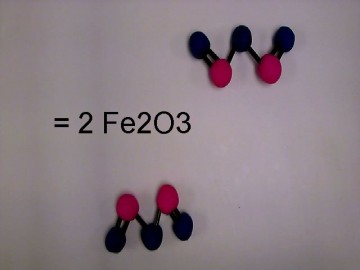Chemical Reaction Using Clay Model Molecules
Prompt
Each student or team picks a simple chemical reaction to recreate using clay model molecules. The molecules can be made with popsicle sticks and colored clay balls. The popsicle sticks represent chemical bonds, and the different colored clay balls represent different atoms. The students take a picture of the unreacted molecules, the breakdown of the molecules, the reorganization of the atoms, and the final molecules post-reaction. The students should write out the chemical reaction equation associated with the recreated reaction and label each picture. If stop motion animation software is available, a HUE animation of the reaction can be made by each student or team.
Authors: Rose Solow and Orian Sneor
Final Project
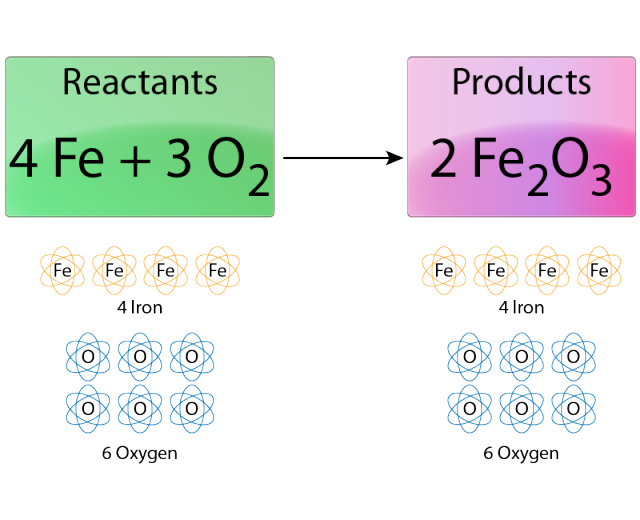
We filmed the chemical reaction of rust using HUE animation. If stop motion animation is not an option students can have a series of pictures in a powerpoint or poster presentation. The students should highlight key features of the molecules and describe the reaction.
Big Idea
By recreating a chemical reaction with model molecules students will be able to:
- Know how to balance a reaction equation
- Recognize the need for the number of atoms to stay the same throughout a chemical reaction
- Know how molecules rearrange during reaction
- Build VSEPR geometries (linear, trigonal planar, tetrahedral etc.)
- Build molecules with single, double, triple bonds
Ask
We wanted a more interactive way for students to learn about chemical reactions. We wanted them to rearrange the atoms themselves for them to get a better idea for how chemicals react and the necessity for balancing reaction equations by realizing the number of atoms needs to remain the same. We decided to have the students build the molecules themselves from whatever materials they had around (clay, popsicle sticks, styrofoam, marshmallows, toothpicks.) We then wanted them to work out the chemical reactions themselves using the molecule models. The students could either take step by step photos of the "reaction" or make a stop motion animation. Key facts and figures concerning the molecules involved should be written or discussed by the students.
Key questions:
- What reaction to replicate?
- What do we use to make the molecules?
- How do we show the reaction?
Brainstorm
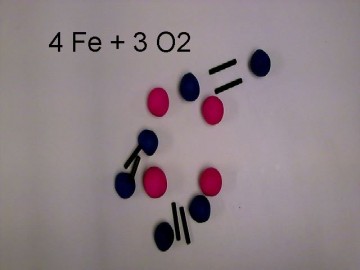
We decided to recreate a common, everyday reaction that can be easily identified. We chose the process of rust. We then balanced the reaction equation to determine the number of molecules necessary. We also wanted to give short descriptions about the molecules involved such as state properties, atomic weight, and scientific name.
Plan
We had clay with different colors available to us so we thought it would be a good idea to use different colored clay balls for the different atoms. We had Lego connect sticks so we chose those to represent our bonds. We decided to use one stick for single bond and two sticks for double bond.
Materials: Clay, Lego connect sticks, camera
Create
We put all of the molecules together and then we filmed the reaction using HUE stop motion animation. We put up the important facts of the molecules on the introductory photos. We also added the reaction equation over the course of the film.
Improve
While the molecules are breaking down, it would be good if we could somehow show their charges or valence electrons. This would be more accurate to the process and would be a more interactive way for the students to understand how chemical reactions occur.