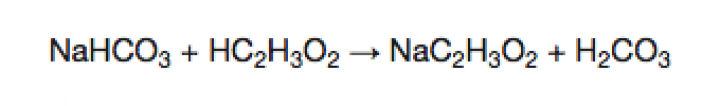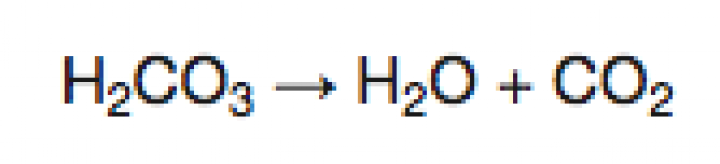Shooting a Projectile Using a Chemical Reaction
Prompt
How far can you shoot a projectile using a chemical reaction? Build a system that shoots some object (ie a cork, ping pong ball, bottle rocket) powered solely by a chemical reaction. Have the students showcase their devices and whoever shoots the projectile highest wins. Examples of chemical reactions include Mentos and Diet Coke, baking soda and vinegar, and heating isopropyl alcohol in an enclosed bottle. The students should describe in writing or during a presentation the science behind the chemistry powering their device, how they built their system, and why they did what they did.
Authors: Rose Solow and Orian Sneor
Final Design
We built a baking soda and vinegar bottle rocket using a plastic soda bottle as the body of the rocket, a cork as the stopper, and pencils to keep it standing. We duct taped three pencils to the outside of the bottle to give it upright stability. We poured vinegar into the bottle and then added a packet of baking soda. Next, we inserted a cork into the mouth of the bottle, shook it up, and placed the rocket on the ground. The vinegar and baking soda reaction produced a build up of gas pressure which propulsed the bottle rocket when that gas escaped.

Big Idea
The goal of this project is to engage students in chemistry. We hope to do this by:
- Making chemistry interesting and exciting
- Involving students in a goal-based engineering project
To complete this project, students must be able to:
- Understand and explain expanding chemical reactions
- Imagine, plan, and build a device that makes use of chemistry to launch something in the air
- Work in groups
Ask
When we were coming up with a design prompt for chemistry, we knew we wanted a project where the students would have to build some kind of device and incorporate their chemistry knowledge into the building of that device. We started with the question, what did we find most exciting about chemistry? The classroom experiments we enjoyed the most when we learned chemistry were examples of chemical reactions. Seeing chemicals react was the best way for us to understand their processes. The most fun chemical reactions for students to watch are reactions involving a significant change, such as rapid color change, expansion, or explosion. Along these lines, we decided that we could have the students use a chemical reaction to propel some object into the air. The students could choose which object to shoot, and how. Students could shoot a small projectile (such as a cork or a small ball) or they could choose to project their entire device (such as a bottle rocket).
Key questions:
- What is the best chemical reaction to propel our object?
- What kind of object do we shoot?
- What kind of device do we build?
Brainstorm
We looked into what common chemicals could be used to produce the energy needed for propulsion. After some quick internet searching, we decided to look into baking soda interacting with vinegar, and Mentos interacting with coke. These two seemed like viable options because they were easily accessible, inexpensive, and their interactions were very propulsive. We also thought of some simple projectiles that we could shoot (cork, marble, soda bottle, ping pong ball.)
Plan
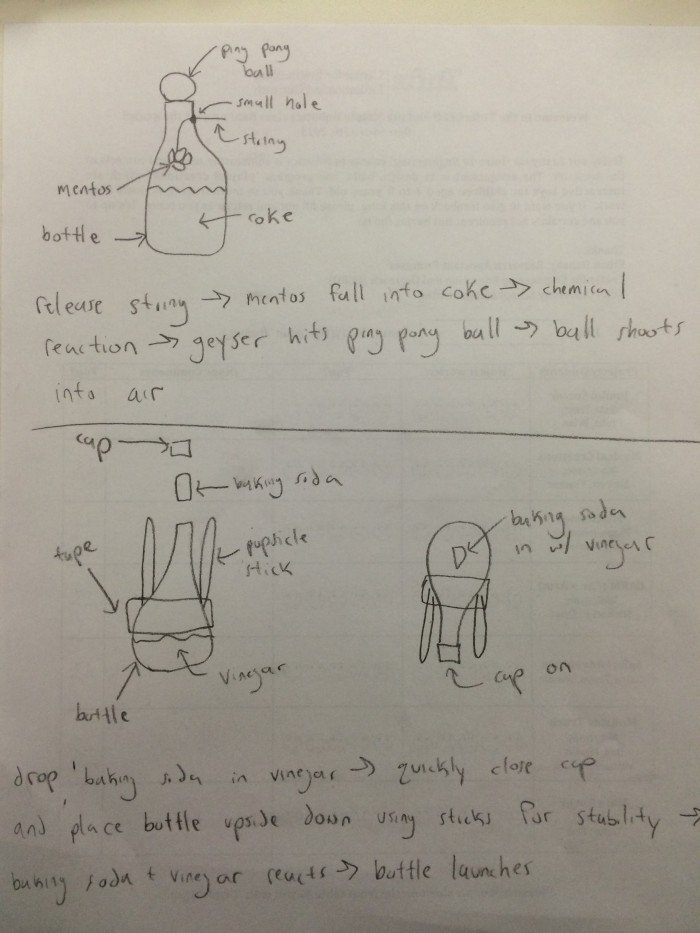
We came up with two ideas for a propulsion device: (1) Mentos and Coke to shoot a ping pong ball out of a coke bottle and (2) baking soda and vinegar to shoot a bottle rocket. We decided to build both sets of devices to showcase both options.
Create and Test the Coke and Mentos Device
First, we built the coke and Mentos device. As we were building, we decided to do away with the string aspect of the design, and instead attach tubing to the top of the bottle and stack the Mentos inside with a popsicle stick underneath that could be pulled out to drop the Mentos into the coke. We cut a small slice into the neck of the bottle to slot the popsicle stick inside and to make it easy to remove for release. We duct taped PVC pipe to the mouth of the bottle to hold the Mentos before release. We then placed a ping pong ball on top of the tube.
Materials: Diet Coke, coke bottle, Mentos, PVC pipe, popsicle stick, duct tape, ping pong ball
In testing, we did not get the response we hoped for. The soda did not shoot dramatically from the top as hoped. Instead, it only fizzed. There are a few possible reasons for this: perhaps we needed more carbon dioxide for the reaction to be extreme, and should have used a bigger bottle of Coke, maybe we should have used more Mentos, maybe the Mentos did not fall into the Coke fast enough, or maybe the pressure escaped through the hole in the side and not out the top.
The Reaction of Coke and Mentos
Technically, the interaction of Coke and Mentos isn't a chemical reaction, but a physical one. The surface of the Mentos is riddled with small pits and crevices which allow for carbon dioxide bubbles to easily form. As the Mentos fall through the soda the bubbles continue to form on the surface and then float up through the liquid. When those bubble form rapidly and at at a great enough quantity, a geyser will burst forth from the liquid.
Create the Baking Soda and Vinegar Bottle Rocket
While building the rocket, we deviated from the original design, and decided that a cork would be a more effective stopper than the screw cap we originally planned. With a cork, the gas could build up and successfully release when it reached too high of a level. We used pencils in lieu of popsicle sticks since they were more readily available. We duct taped the pencils to the bottle to act as stands for the rocket. We poured vinegar into the bottle and rolled some baking powder in a piece of paper to add to the bottle during launch. The plan was to drop the baking powder packet into the bottle, put the cork on, shake the rocket up, put it on the ground, and watch it explode.
Reference video here
Materials: Pencils, soda bottle, cork, duct tape, paper, vinegar, baking soda
Test Baking Soda and Vinegar Bottle Rocket Launch
Launching the bottle rocket at first proved difficult. We had problems with the baking soda packet releasing the soda to allow for the reaction to take place. Unfortunately, our efforts did not go as planned. We tried over and over again, but either the baking soda reacted to quickly and the cork would not go in, or the baking soda reacted too slowly and then all at once and the rocket blew up in our hands. Finally, on the fifth try, it worked. We decided that white paper was too thick and we then used a napkin. We still had to shake the bottle a little before launch.
The Chemical Reaction of Baking Soda and Vinegar
When baking soda and vinegar interact, they begin a two step chemical reaction. The acetic acid of vinegar reacts with the sodium bicarbonate of baking soda to form carbonic acid and sodium acetate. Carbonic acid is an unstable molecule and after the initial reaction it begins to break down. The carbonic acid decomposes into water and carbon dioxide gas. When this reaction occurs in a closed container with a fixed volume, the production of the carbon dioxide gas increases the internal pressure. In the case of our bottle rocket, that internal pressure eventually dislodges the cork and releases that pressure build up, propelling the rocket upward.
Improve
We tested both devices, and the baking soda and vinegar bottle rocket was much more successful than the Coke and Mentos device. We believe that the Coke and Mentos device would be vastly improved if we used a 2 L bottle rather than a 16 oz bottle to increase the amount of carbon dioxide in the interaction, and did not make a slit in the neck of the bottle, which let a lot of pressure escape. The bottle rocket worked successfully, but we had to go through a few iterations of paper packet choices (thinner rather than thicker paper) for the baking soda before it was completely successful.